How to meet ISO 9001-2015 Documentation Requirements
Like most standards, ISO 9001:2015 only tells you what to do (the requirements), but not how to do it. While this gives organizations the freedom to meet the requirements in whatever ways they deem useful and efficient, a lack of specific guidance can also be overwhelming and confusing. This implementation freedom is particularly relevant with the changes in documentation requirements for ISO 9001:2015.
Your Organization of Documentation Will Change
ISO 9001:2015 has changed from 8 to 10 sections in order to standardize quality requirements across a variety of standards, following a new “high level” format called Annex L. Several of the requirements were shuffled in this change, so you’ll end up with a new structure in your QMS that an auditor will have to follow. But because the new format is now consistent across many ISO management systems standards, it is worth investing time in conforming to the structure since it will likely be around a while.
“Analysis of the processes should be the driving force for defining the amount of
documented information needed for the quality management system, taking into
account the requirements of ISO 9001:2015. It should not be the documented
information that drives the processes.”
Source: ISO Guidance on the requirements for Documented Information for ISO 9001:2015
Documentation Approaches Can Vary
We’ve advised on identifying what types of information to document in the new ISO 9001:2015 QMS. But how is this to be implemented? Documentation approaches can vary widely. As an example, the word “Quality Manual” means different things to different people:
- Example 1: A simple quality requirements manual that doesn’t include any specific procedures.
- Example 2: A manual with requirements and procedures in one document.
- Example 3: A manual addressing the requirements in an abridged fashion along then referring to procedures for more detailed instruction
The first option doesn’t provide any information about how a company controls the processes, and these details will need to be documented elsewhere.
The second option, a manual with procedures listed all in one document, will often be too large to comprehend easily and manage for efficient document control. When a small change regarding one part of the organization needs to be made, the entire manual will require updating – even if the change is irrelevant to most areas.
The third option addressed each requirement in a quality manual then referred to separate individual procedures. This was primarily because ISO 9001:2008 required a manual and procedures. In this case, simple changes can be made to specific areas of the QMS without having to update the entire QMS.
However, the ISO 9001:2015 Standard allows a more efficient way to manage documented information: Cover the Organization wide requirements (Context of the organization, interested parties, etc) in a small document, then defer to individual procedures to address the requirements for the various processes. This allows the flexibility to only address the requirements within your scope and eliminate duplication of topics in a manual and a procedure.
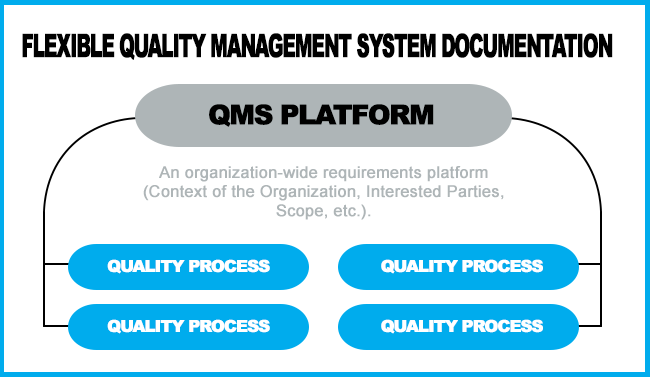
What to Consider as You Organize Your Documentation
Because ISO 9001:2015 does not mandate a Quality Manual and six procedures as it did before, documented information should vary based on the size and need of an organization. This will allow great flexibility to use software, flowcharts, papers, photographs, videos, digital files, as allowed by the new ISO 9001 revision.
Thus, an ISO 9001:2015 QMS essentially says you need “documented information” for all processes in your business. And while the updated standard allows this documentation be structured in a less prescriptive manner, really nothing has changed: You still need to document the processes, records, etc. related to operating your organization.
But there are still a few things to consider as you lay out your documentation under the new guidelines, for example, your documentation should be:
- Easy to follow – Auditors appreciate following an organized QMS.
- Flexible – If the scope of your QMS changes, does it cause upheaval in all your documentation?
- Scalable – What if you decide to achieve certification in additional standards like ISO 14001? How would this affect your documentation?
- Structured using the process approach – ISO recommends using the process approach to document processes to the extent required to ensure their “effective operation and control.”
Is there still a place for the quality manual?
It makes sense to create a foundational main manual that summarizes the organization-wide issues as they relate to quality. This would include sections 1-4 of the standard:
- Scope
- Normative References
- Terms and Definitions
- Context of the Organization.
As noted above, a quality manual, can also serve as a platform to index the entire scope of the QMS.
To retain flexibility, our experience led us to move all other requirements to individual procedures, making it easier for individual organizations to include/exclude the processes that are relative to the scope of their QMS. The main manual sets the stage for most of the requirements addressed in the procedures and work instructions. Keeping the procedures as separate documents allows for more efficient document control that you would have with one large manual needing to be redistributed to all portions of the facility if only one area changes.
Furthermore, should you choose to integrate any other standards (ISO 14001:2015, for example), this platform allows for:
- One, simple, consolidated manual
- Shared procedures (when possible)
- A scalable format
Integrated management systems offer organizations the benefit of increased efficiency and effectiveness and cost reductions. A QMS format that accommodates integration with other types of management systems (EMS/SMS/etc) offers your company more flexibility in the future.
Don’t Re-invent the Wheel
This approach to quality documentation is different than what many are used to, but, overall, it is quite efficient, flexible, and scalable – providing you a great platform for future changes in your organization.
But just because it is different, doesn’t mean it has to be difficult to implement. Organizations with an existing QMS need not get rid of all of their existing documented information, especially if they structured their QMS using a process approach. If you are an organization building your QMS for the first time, take advantage of templates and other resources used by similar businesses. You can modify with processes and procedures specific to your company. Standards Stores offers a fully “customizable” ISO 9001:2015 Quality Manual and Procedures package with a variety of templates to get you started creating a foundational quality policy and facilitate a simpler, speedier transition, ultimately saving you time and money. Our system is the result of continual improvement of processes and procedures over the last decade, and all of our products are backed by our guarantee.


